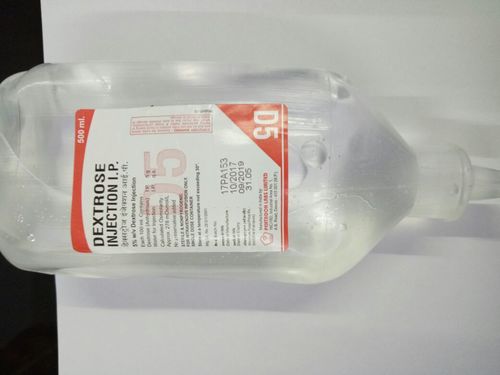
Dextrose Injection
18 INR
Product Details:
- Drug Type Injection
- Physical Form Liquid
- Recommended For Carbohydrate Calories
- Suitable For Adults
- Click to view more
X
Dextrose Injection Price And Quantity
- 10000 Piece
- 18 INR
Dextrose Injection Product Specifications
- Injection
- Adults
- Liquid
- Carbohydrate Calories
Dextrose Injection Trade Information
- Cash in Advance (CID) Cash Advance (CA)
- 1 Piece Lac Per Week
- 1 Week
- Australia Central America North America Eastern Europe Western Europe Middle East Africa South America Asia
Product Description
5% Dextrose Injection, USP solution is sterile and nonpyrogenic. It is a parenteral solution containing dextrose in water for injection intended for intravenous administration.Each 100 mL of 5% Dextrose Injection, USP, contains dextrose monohydrate, 5 g in water for injection. The caloric value is 170 kcal/L. The osmolarity is 252 mOsmol/L (calc.), which is slightly hypotonic.
his solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
5% Dextrose Injection, USP is a parenteral fluid and nutrient replenisher.
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 H2O), a hexose sugar freely soluble in water. It has the following structural formula:
CLINICAL PHARMACOLOGY:
When administered intravenously, these solutions provide a source of water and carbohydrate.
Isotonic and hypertonic concentrations of dextrose are suitable for parenteral maintenance of water requirements when salt is not needed or should be avoided.
INDICATIONS AND USAGE:
Intravenous solutions containing dextrose are indicated for parenteral replenishment of fluid and minimal carbohydrate calories as required by the clinical condition of the patient.
CONTRAINDICATIONS:
5% Dextrose Injection, USP without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility that pseudoagglutination of red cells may occur.
WARNINGS:
Excessive administration of potassium-free solutions may result in significant hypokalemia.
The intravenous administration of these solutions can cause fluid an
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Pharmaceutical Injectables' category
"Accepting orders in bulk order quantity".
 |
DHEER HEALTHCARE PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |